Abstract
Background: Among clinicians, it is well-known that the identification of a monoclonal(M) protein may disappear at a later timepoint (transient M protein) after certain infections, in the setting of some automimmune conditions, or other conditions. However, there is no information from a systematic study investigating patterns of transient M-proteins in a prospectively monitored cohort.The aim of this large, population-based study was to describe the epidemiology and characteristics of individuals with transient M-proteins in the prospective screening manner based on the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study. Furthermore, given the recent introduction of highly sensitive mass spectrometry (MS) based assays for the identification and quantification of M proteins, we were motivated to investigate whether transience is also related to an increased sensitivity of the assay used in a comparison with conventional serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE).
Methods: The iStopMM study is an ongoing nationwide screening study for multiple myeloma precursors in Iceland. A total of 75,422 Icelanders ≥40 years old (51% of the population) were screened for M protein using SPEP and free light chains. Participants with a detectable M-protein entered a randomized controlled trial with three arms where arms 2 and 3 underwent initial testing and longitudinal follow-up. The transient M-protein cohort was defined as individuals with a positive SPEP who at a later timepoint returned a negative SPEP/IFE sample. Comorbidity data and prescription medicine use was acquired from three high-quality national disease and prescription medicine registries. MS (EXENT®, Binding Site, Birmingham, UK) analyses were performed on the original diagnostic sample and a follow-up sample with a cutoff of 0,02g/L considered as positive. We evaluated comorbidity and medication use comparing individuals with transient M protein to age- and sex-matched subjects with persistent M protein in a 4:1 matched nested case-control study. Differences between the groups were analyzed with chi square tests.
Results: Overall, the iStopMM MGUS cohort comprises 3,358 individuals. Of those 2,037 underwent annual follow-up testing. A total of 198 (9.8%) individuals were identified as having transient M protein according to SPEP and IFE.
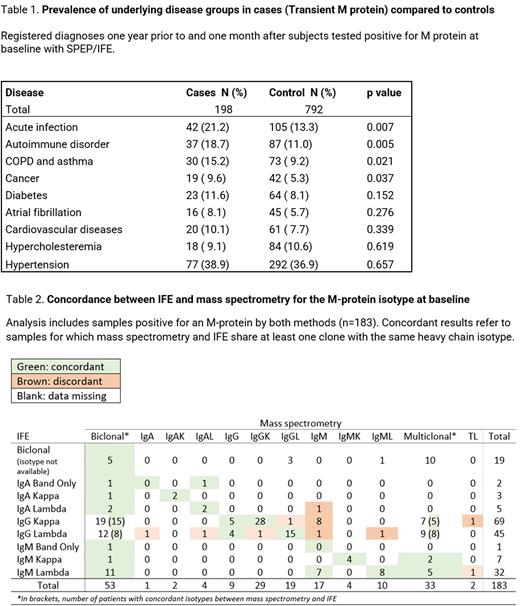
In the transient M protein cohort we observed an increased prevalence of infections, autoimmune disorders, cancer, and obstructive pulmonary conditions (Table 1). We even observed increased usage of corticosteroids (17.7% vs 9.8%; p = 0.01), immunosuppressive medications (5.6% vs 2.2%; p=0.02), azithromycin (9.6% vs 4.9%; p=0.02), and diuretics (20.7% vs 13.3%; p=0.03). These drugs were found to be given between one year prior to one month after the subjects tested negative for M protein with SPEP/IFE.
Among 198 individuals reported to have transient M protein according to SPEP and IFE, baseline MS samples were positive at in 183 (92%). The remaining 15 (8%) did not have an identifiable M protein on MS at baseline. Twelve (80%) of these 15 individuals had an active infection, autoimmune disorder, or active cancer at the initial measurement.
Of the 183 individuals who had M protein identified at baseline by both SPEP/IFE and MS, MS identified the same M protein in 70% (129/183) of the cases at follow-up when SPEP/IFE was negative.
Fifty four individuals were observed to be MS positive at baseline and negative at a follow-up testing indicating a prevalence of transient M-proteins of 2.6% within a cohort of MGUS patients when measured by MS. MS showed good concordance with IFE (Table 1), and, as expected, use of more sensitive MS in baseline samples results in additional increase by 33% in low-concentration M protein spikes, compared to conventional SPEP/IFE.
Conclusion: In this large prospective study we found that M proteins were transient in almost 10% of cases. Using a more sensitive MS, the proportion of transient M proteins was only 2.6%. Thus, most of individuals with transient M proteins based on SPEP/IFE are falsely negative, when using MS as the benchmark. Our prospective cohort study provides novel insights on longitudinal patterns of abnormal serum proteins, it highlights how the use of MS results in the identification of low M proteins, and it shows that transient M proteins are often only below the limit of detection with conventional SPEP/IFE.
Disclosures
Berlanga:The Binding Site: Current Employment. Onundarson:Pall T. Onundarson MD: Patents & Royalties: patent for a new coagulation test, the Fiix prothrombin time. Hultcrantz:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Curio Science LLC: Consultancy; Intellisphere LLC: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen, Daichii Sankyo, Cosette, GSK: Research Funding. Landgren:Leukemia & Lymphoma Society: Research Funding; Rising Tide Foundation: Research Funding; Tow Foundation: Research Funding; MMRF: Honoraria; Aptitude Health: Honoraria; Riney Foundation: Research Funding; Merck & Co., Inc.: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Pfizer Inc: Consultancy; Theradex: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; NCI/NIH: Research Funding; Janssen: Honoraria, Other: Independent Data Monitoring Committee (IDMC) member for clinical trials, Research Funding; Amgen: Honoraria, Research Funding. Harding:The Binding Site: Current Employment, Membership on an entity's Board of Directors or advisory committees. Durie:Amgen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Kristinsson:Celgene: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal